A layer of titanium dioxide (titania: TiO2) that forms on titanium (Ti) metal improves its corrosion resistance and biocompatibility and provides photocatalytic activity. The polymorphic phases of TiO2 are rutile, anatase, and brookite. On a macroscopic scale, rutile is thermodynamically stable relative to anatase and brookite. It has been reported that an anatase phase formed on Ti implants improves their bone compatibility. In addition, anatase as well as anatase-rutile and anatase-brookite composites are known to exhibit high levels of photocatalytic activity. Thermal oxidation using gas is a simple and low-cost method to prepare a TiO2 layer with high adherence to Ti substrates and can be applied to substrates with a complex geometry. Understanding and controlling the TiO2 phases on Ti by thermal oxidation contributes to expanding the applications of Ti in biomedical and photocatalytic fields. Many studies have been conducted on the formation of an anatase-rich TiO2 layer during the thermal oxidation of TiC and TiN. From these reports, it is expected that an anatase-rich TiO2 layer will be obtained by the combination of the formation of Ti![]() C or Ti
C or Ti![]() N system products on Ti and the subsequent oxidation of these products. In this study, a two-step gas treatment involving carburization or nitridation and oxidation in air was investigated for the formation of an anatase-rich TiO2 layer on Ti.
N system products on Ti and the subsequent oxidation of these products. In this study, a two-step gas treatment involving carburization or nitridation and oxidation in air was investigated for the formation of an anatase-rich TiO2 layer on Ti.
Commercially pure (CP) Ti plates (JIS Gr. 2) with dimensions of 10 ![]() 10
10 ![]() 2 mm were used as specimens for the two-step gas treatment. Two kinds of conditions were employed for the first step: Ar
2 mm were used as specimens for the two-step gas treatment. Two kinds of conditions were employed for the first step: Ar![]() 1%CO atmosphere at 1073 K for 0 ks and N2 atmosphere at 1123 K for 14.4 ks. The gas flow rates were 6.7
1%CO atmosphere at 1073 K for 0 ks and N2 atmosphere at 1123 K for 14.4 ks. The gas flow rates were 6.7 ![]() 10-6 m3·s-1 and 3.4
10-6 m3·s-1 and 3.4 ![]() 10-6 m3·s-1 at the standard state for Ar
10-6 m3·s-1 at the standard state for Ar![]() 1%CO and N2 atmospheres, respectively. The total gas pressure of both the atmospheres was 0.1 MPa. The first step was carried out using an electric resistance furnace with a silica reaction tube. Specimens were set into the furnace at room temperature and heated at a rate of 0.14 K·s-1. The gas atmosphere during the increases and decreases in the specimen temperature was kept the same as that during holding. The second step was carried out in air at 473
1%CO and N2 atmospheres, respectively. The total gas pressure of both the atmospheres was 0.1 MPa. The first step was carried out using an electric resistance furnace with a silica reaction tube. Specimens were set into the furnace at room temperature and heated at a rate of 0.14 K·s-1. The gas atmosphere during the increases and decreases in the specimen temperature was kept the same as that during holding. The second step was carried out in air at 473![]() 873 K for 0 ks and 86.4 ks using an electric resistance muffle furnace after the first step. The specimen temperature was increased to a specified value at a rate of 0.14 K·s-1 and cooled in the furnace. After the first and second steps, the reaction layer on Ti was analyzed by
873 K for 0 ks and 86.4 ks using an electric resistance muffle furnace after the first step. The specimen temperature was increased to a specified value at a rate of 0.14 K·s-1 and cooled in the furnace. After the first and second steps, the reaction layer on Ti was analyzed by ![]() -2
-2![]() X-ray diffraction (XRD) with an incident angle of 0.3
X-ray diffraction (XRD) with an incident angle of 0.3![]() and X-ray photoelectron spectroscopy (XPS). Cross sections of the reaction layers were observed by scanning electron microscopy (SEM), and their thicknesses were measured.
and X-ray photoelectron spectroscopy (XPS). Cross sections of the reaction layers were observed by scanning electron microscopy (SEM), and their thicknesses were measured.
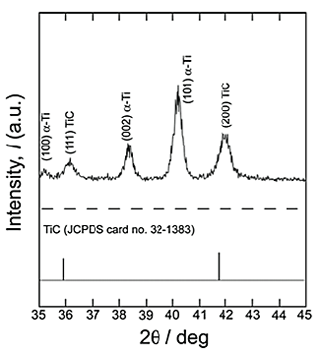
Figure 1 shows the ![]() -2
-2![]() XRD pattern of reaction layer after the first-step treatment in Ar
XRD pattern of reaction layer after the first-step treatment in Ar![]() 1%CO atmosphere at 1073 K for 0 ks. The XRD pattern of the reaction layer was close to the standard pattern of TiC, although the reflections shifted toward higher angles. The presence of both Ti
1%CO atmosphere at 1073 K for 0 ks. The XRD pattern of the reaction layer was close to the standard pattern of TiC, although the reflections shifted toward higher angles. The presence of both Ti![]() C and Ti
C and Ti![]() O bonds was confirmed by XPS analyses. Therefore, we concluded that the phase in reaction layer was titanium oxycarbide, TiC1-xOx. The lattice constant of TiC1-xOx is reported to be less than that of TiC; this is consistent with the shift in the reflections shown in Figure 1. The thickness of TiC1-xOx layer was around 100 nm, which was measured by the SEM observation. Figure 2 shows the
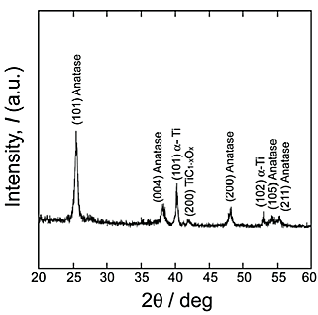
O bonds was confirmed by XPS analyses. Therefore, we concluded that the phase in reaction layer was titanium oxycarbide, TiC1-xOx. The lattice constant of TiC1-xOx is reported to be less than that of TiC; this is consistent with the shift in the reflections shown in Figure 1. The thickness of TiC1-xOx layer was around 100 nm, which was measured by the SEM observation. Figure 2 shows the ![]() -2
-2![]() XRD pattern of reaction layer after the second step in air at 573 K for 86.4 ks, with the first-step treatment in Ar
XRD pattern of reaction layer after the second step in air at 573 K for 86.4 ks, with the first-step treatment in Ar![]() 1%CO atmosphere. The anatase phase formed as a reaction product, and a small amount of the TiC1-xOx phase was detected. Since the rutile phase was not detected in the reaction layer, the TiO2 layer formed on Ti under this condition was single-phase anatase. Figure 3 summarizes the phases of reaction layer formed on Ti after the second step in air at 473
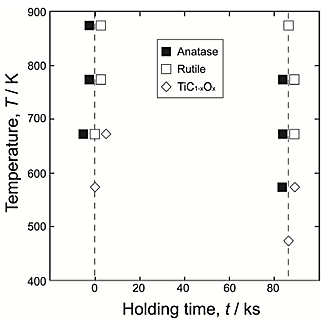
1%CO atmosphere. The anatase phase formed as a reaction product, and a small amount of the TiC1-xOx phase was detected. Since the rutile phase was not detected in the reaction layer, the TiO2 layer formed on Ti under this condition was single-phase anatase. Figure 3 summarizes the phases of reaction layer formed on Ti after the second step in air at 473![]() 873 K for 0 ks and 86.4 ks, with the first-step treatment in Ar
873 K for 0 ks and 86.4 ks, with the first-step treatment in Ar![]() 1%CO atmosphere. An anatase-rutile composite layer was obtained under a wide range of conditions for the second step. As shown in Figure 3, the TiC1-xOx phase was not fully oxidized under the conditions at 573 K for 86.4 ks and 673 K for 0 ks. Figure 4 shows the phase fraction in reaction layers after the second step in air at 473
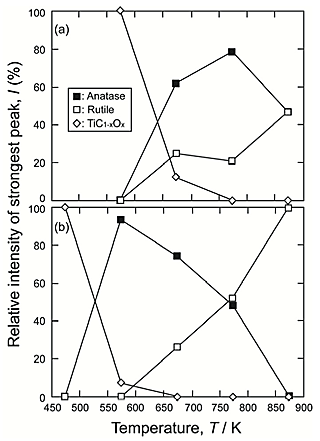
1%CO atmosphere. An anatase-rutile composite layer was obtained under a wide range of conditions for the second step. As shown in Figure 3, the TiC1-xOx phase was not fully oxidized under the conditions at 573 K for 86.4 ks and 673 K for 0 ks. Figure 4 shows the phase fraction in reaction layers after the second step in air at 473![]() 873 K for 0 ks and 86.4 ks in terms of the relative intensities of the strongest peaks of phases in the
873 K for 0 ks and 86.4 ks in terms of the relative intensities of the strongest peaks of phases in the ![]() -2
-2![]() XRD patterns. An anatase-rich layer formed on Ti under the second-step conditions at 573
XRD patterns. An anatase-rich layer formed on Ti under the second-step conditions at 573![]() 773 K, depending on the holding time. It is expected that the anatase fraction in reaction layer will be controlled by varying the heating temperature and holding time of the second step.
773 K, depending on the holding time. It is expected that the anatase fraction in reaction layer will be controlled by varying the heating temperature and holding time of the second step.
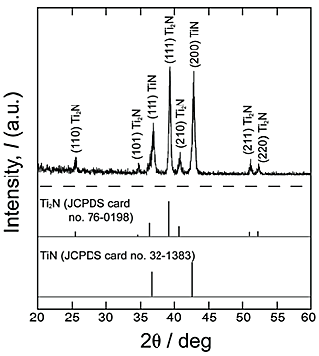
Figure 5 shows the ![]() -2
-2![]() XRD pattern of reaction layer after the first-step treatment in N2 atmosphere at 1123 K for 14.4 ks. TiN and Ti2N phases were detected, and Ti2N was a major phase in the reaction layer. Since the Ti2N was the major phase in nitride layer obtained in this study, even in the result of the
XRD pattern of reaction layer after the first-step treatment in N2 atmosphere at 1123 K for 14.4 ks. TiN and Ti2N phases were detected, and Ti2N was a major phase in the reaction layer. Since the Ti2N was the major phase in nitride layer obtained in this study, even in the result of the ![]() -2
-2![]() XRD measurement, it is speculated that the thin TiN layer forms on the surface of the reaction layer. Figure 6 shows the
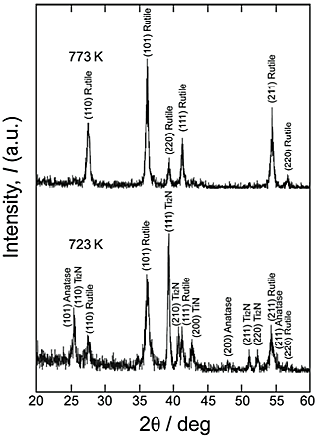
XRD measurement, it is speculated that the thin TiN layer forms on the surface of the reaction layer. Figure 6 shows the ![]() -2
-2![]() XRD patterns of reaction layer after the second step in air at 723 K and 773 K for 86.4 ks, with the first-step treatment in N2 atmosphere. Anatase and rutile phases formed after the second step at 723 K for 86.4 ks, and only the rutile phase was detected at 773 K for 86.4 ks. It has been reported that the first oxide detected on oxidation scale of TiN after oxidation in air was an anatase-rutile phase and that the amount of anatase in the scale was diminished with an increase in the heating temperature. After carrying out the second step at 773 K for 86.4 ks, only the rutile phase was formed and neither a TiN nor a Ti2N phase was detected. This indicated that both nitrides formed on Ti were oxidized to the rutile phase. A TiO2 layer formed after the second step at 723 K for 86.4 ks contained an anatase phase but was rutile rich. Since the formation of an anatase layer on Ti by thermal oxidation via nitridation has the possibility of improving the photocatalytic activity of the anatase layer in the visible region, our future plan is to form an anatase-rich TiO2 layer on Ti by the two-step gas treatment via nitridation.
XRD patterns of reaction layer after the second step in air at 723 K and 773 K for 86.4 ks, with the first-step treatment in N2 atmosphere. Anatase and rutile phases formed after the second step at 723 K for 86.4 ks, and only the rutile phase was detected at 773 K for 86.4 ks. It has been reported that the first oxide detected on oxidation scale of TiN after oxidation in air was an anatase-rutile phase and that the amount of anatase in the scale was diminished with an increase in the heating temperature. After carrying out the second step at 773 K for 86.4 ks, only the rutile phase was formed and neither a TiN nor a Ti2N phase was detected. This indicated that both nitrides formed on Ti were oxidized to the rutile phase. A TiO2 layer formed after the second step at 723 K for 86.4 ks contained an anatase phase but was rutile rich. Since the formation of an anatase layer on Ti by thermal oxidation via nitridation has the possibility of improving the photocatalytic activity of the anatase layer in the visible region, our future plan is to form an anatase-rich TiO2 layer on Ti by the two-step gas treatment via nitridation.