Effects of solute Fe atoms in Al on material properties had been considered to be negligible because the maximum solid solubility that can be dissolved in Al lattice is quite low (0.026 at.% Fe). Thus, the strengthening effect through Fe had been assumed to be only from the presence of Al3Fe precipitates. However, recently it has been reported that creep resistance of high-purity Al was significantly enhanced by dilute Fe addition in solid solution. The steady-state creep rate of Al-0.026 at.% Fe alloy containing about 0.02 at.% Fe in solid solution was 1000 times slower than that of high-purity Al at 673 K. However, these results exactly included the effect of not only solute Fe atoms but also precipitates because Fe content exceeded the solubility limit. Therefore, in the present study, to have detailed understandings of effect of solute Fe atoms on high temperature deformation of Al, creep tests of ultra-high-purity (99.999%) Al and Al-Fe solid solution alloys whose solute concentrations are lower than the solubility limit were conducted.
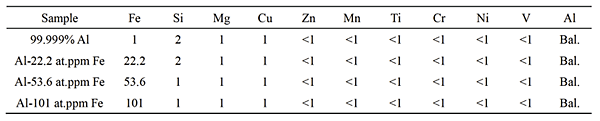
The samples used in the present study were 99.999% Al and three kinds of Al-Fe binary alloys. The contents of Fe were 22.2, 53.6 and 101 at.ppm Fe which were lower than the maximum solubility limit of Fe in Al, 260 at.ppm. The chemical compositions are listed in Table 1. The samples were solutionized for 4 h at 913 K. Tensile creep tests were carried out at 773 K in the stress range of 2-6 MPa.
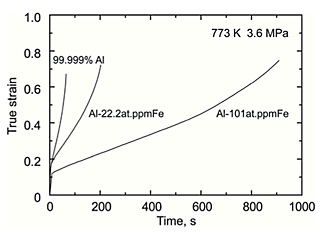
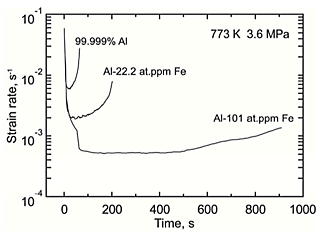
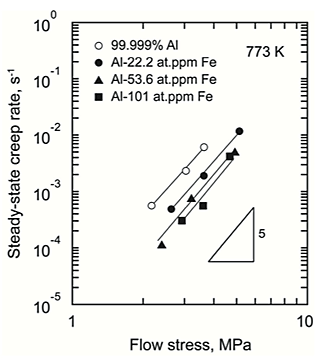
Figure. 1 shows the typical creep curves for 99.999% Al, Al-22.2 at.ppm Fe and 101 at.ppm Fe at 3.6 MPa at 773 K taken to rupture. Creep resistance of 99.999% Al was enhanced by dilute Fe addition. Figure. 2 shows the variation in the strain rate as a function of the time. Primary creep stage followed by secondary and ternary creep stage were clearly observed. After the primary creep stage, the strain rate reached a constant creep rate and then accelerated with increasing the strain. Figure. 3 shows the variation in the steady-state creep rate, calculated from the average of the strain rate at the secondary creep stage, as a function of the flow stress. Al-101 at.ppm Fe alloy is seen to creep by a factor of about 10 times slower than 99.999% Al. The stress exponents exhibited values of about 5. This result indicates that climb-controlled dislocation creep was a dominant deformation mechanism.
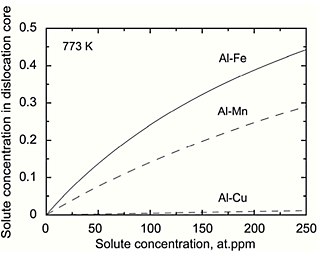
Figure. 4 shows the relationship between solute concentration in dislocation core, which were obtained by using the solute-dislocation interaction energy calculated from the first principles energy, and solute concentration for Al-X (X = Fe, Mn and Cu) binary solid solution alloys at 773 K. Solute Fe concentration in dislocation core is higher than any other solute spices. Therefore, it could be suggested that Fe atoms segregating in dislocations due to the strong interaction between solute Fe atoms and the dislocation enhanced the creep resistance.