Nickel-titanium (NiTi) alloys have excellent superelastic properties and shape memory effects with a recovery strain of 10%; they are used commercially in many fields such as aerospace, biomedical, and automotive because they also exhibit superior mechanical strength, ductility, corrosion resistance, and workability. NiTi alloy ingots are industrially manufactured by induction melting with graphite and oxide crucibles, from which impurity carbon and oxygen are introduced into the NiTi alloy melts during the melting process. Lime (CaO) is one of the candidate oxide crucible materials in the induction melting of NiTi alloys. The introduction of carbon into NiTi alloy melts can be suppressed during melting in a CaO crucible, but oxygen will be introduced. Therefore, the development of an effective deoxidation process for NiTi alloy melts, using a CaO crucible to control the oxygen content in NiTi alloy components, is necessary. Metallic barium (Ba) has high thermodynamic affinity with oxygen and was reported to be effective for deoxidation of iron melts in a CaO crucible. The objectives of this study were to investigate Ba deoxidation of NiTi alloy melts and to clarify the phase and content of the precipitates in the NiTi alloys before and after the Ba deoxidation.
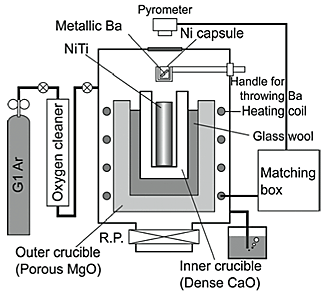
Two types of NiTi alloy bars with initial oxygen contents of 305 ppm (forged bar with a diameter of 20 mm, Low O) and 615 ppm (hot-rolled bar with a diameter of 7.8 mm, High O) were used as the raw materials. The chemical composition of the NiTi alloy bars is shown in Table 1. The alloy was melted in a dense CaO crucible (>99.9%) using an induction furnace, schematically shown in Fig. 1. The melting was carried out at a temperature of 1673 K in Ar atmosphere (gas flow rate of 1.7 ![]() 10-6 m3·s-1 and a total gas pressure of 0.1 MPa). 0.6 g of metallic Ba (>99.9%), that is 1.5 mass% of the NiTi alloy melts, was placed in a Ni capsule. The metallic Ba with the Ni capsule was dropped from the upper part of the chamber of the furnace into the NiTi alloy melt held in the CaO crucible (see Fig. 1) 60 s after reaching the temperature of 1673 K, then the melt was held for a specified period of time ranging between 60 s and 600 s, followed by furnace cooling. The oxygen content in the cooled NiTi alloys was determined by the inert gas fusion-IR absorption method. The Ba and Ca contents in the cooled NiTi alloys were quantitatively measured by inductively coupled plasma-mass spectroscopy (ICP-MS). The microstructures of the cooled NiTi alloys were observed using a scanning electron microscope (SEM). A field emission electron-probe micro analyzer (FE-EPMA) was used for the compositional analyses of the metallic matrix and precipitates. The precipitates in the cooled NiTi alloys were electrolytically extracted in order to precisely analyze their phase and morphology.
10-6 m3·s-1 and a total gas pressure of 0.1 MPa). 0.6 g of metallic Ba (>99.9%), that is 1.5 mass% of the NiTi alloy melts, was placed in a Ni capsule. The metallic Ba with the Ni capsule was dropped from the upper part of the chamber of the furnace into the NiTi alloy melt held in the CaO crucible (see Fig. 1) 60 s after reaching the temperature of 1673 K, then the melt was held for a specified period of time ranging between 60 s and 600 s, followed by furnace cooling. The oxygen content in the cooled NiTi alloys was determined by the inert gas fusion-IR absorption method. The Ba and Ca contents in the cooled NiTi alloys were quantitatively measured by inductively coupled plasma-mass spectroscopy (ICP-MS). The microstructures of the cooled NiTi alloys were observed using a scanning electron microscope (SEM). A field emission electron-probe micro analyzer (FE-EPMA) was used for the compositional analyses of the metallic matrix and precipitates. The precipitates in the cooled NiTi alloys were electrolytically extracted in order to precisely analyze their phase and morphology.
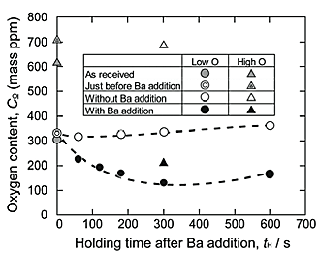
Figure 2 shows the relationship between the holding period after the Ba addition and the oxygen content of the Low O and High O NiTi alloy melts in a CaO crucible at 1673 K. The oxygen contents of the as-received NiTi alloys and the NiTi alloy melted without the addition of Ba are depicted in this figure as well. The oxygen contents were around 350 ppm and 700 ppm in the Low O and High O NiTi alloy melts, respectively, for the specimens with no Ba addition. It is considered that oxygen was introduced into the NiTi alloy melts by the dissolution of CaO, as shown in Eq. (1).
| CaO(s) = Ca(in NiTi melt) + O(in NiTi melt) | (1) |
When Ba was added to the alloy melt, the oxygen contents decreased to 130 ppm and to 210 ppm in the Low O and High O NiTi alloy melts, respectively. This decrease in oxygen content was caused by the reaction of dissolved Ba with the solute oxygen in the NiTi alloy melts, as described in Eq. (2).
| Ba(in NiTi melt) + O(in NiTi melt) = BaO(s) | (2) |
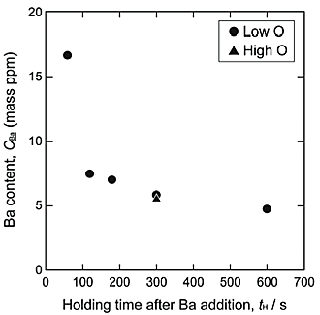
The deoxidation product is considered to be BaO. Figure 3 shows the Ba content in the NiTi alloy melts as a function of the holding period after the Ba addition. The Ba content was less than 10 ppm, 120 s after the Ba addition, regardless of the initial oxygen contents. The analytical values of Ba obtained using ICP-MS represent the total Ba content in the cooled NiTi alloys. Therefore, the results suggest that the deoxidation product, BaO, formed by the reaction shown in Eq. (2), was removed from the melts in a very short holding period such as 120 s.
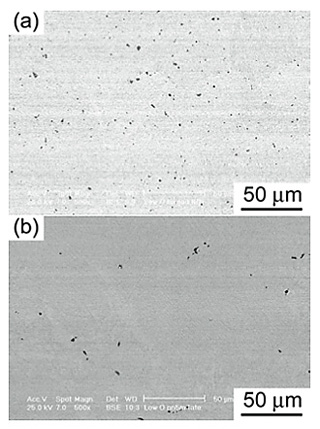
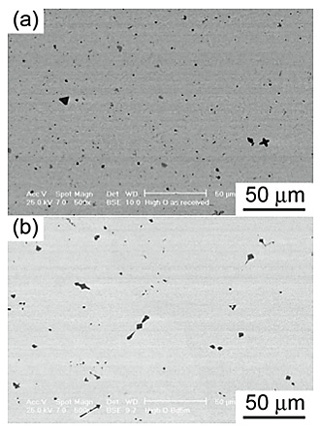
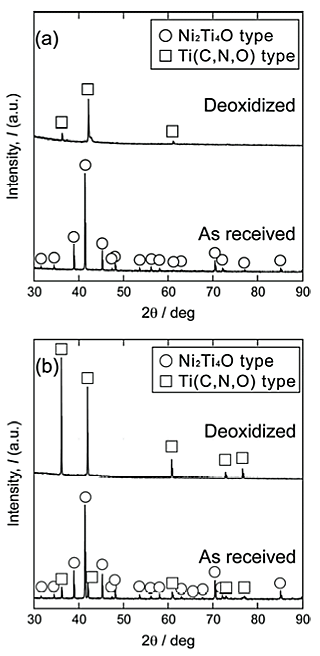
Figures 4 and 5 show the microstructures of the Low O and High O NiTi alloys, respectively, before and after Ba deoxidation. The precipitate contents in the Low O and High O NiTi alloys, calculated using SEM-BSE images, decreased from 1.75% to 0.19% and from 2.48% to 1.44%, respectively, due to the Ba deoxidation. The shape of the precipitates was polygon, blocky, and needle-like. Figure 6 shows the XRD patterns of the precipitates electrolytically extracted from the Low O and High O NiTi alloys cooled in a CaO crucible 300 s after the Ba addition. The phase of the precipitates in the as-received Low O NiTi alloy was single phase of Ni2Ti4O type, while Ni2Ti4O and Ti(C,N,O) type precipitates were detected in the as-received High O NiTi alloy. After the Ba deoxidation, however, a single phase of Ti(C,N,O) type precipitates was observed for both NiTi alloys. The carbon content in the Ti(C,N,O) type precipitates of the High O NiTi alloy was higher than that in the Low O NiTi alloy, which appeared to be caused by the high carbon contents in the raw materials (see Table 1). The present results revealed that the phase of the precipitates depended on both the oxygen and carbon contents in the NiTi alloys. Three precipitate phase regions, Ni2Ti4O type, Ni2Ti4O and Ti(C,N,O) types, and Ti(C,N,O) type, were observed as functions of both oxygen and carbon contents in the NiTi alloys.