Porous aluminum has received much attention in various industrial fields due to their high energy absorptivity together with their superior lightweight. In this study, using FSP (Friction Stir Processing) route precursor method and aluminum alloy die casting ADC12, the fabrication possibility of the porous aluminum changing the pore structures in one individual was discussed.
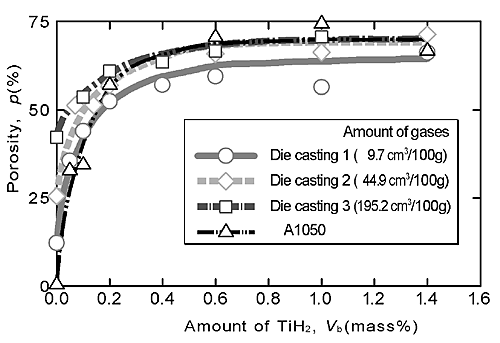
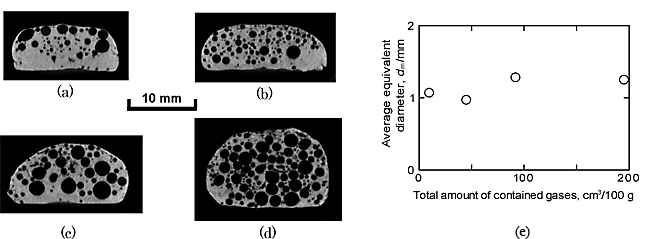
Figure 1 shows the relationship between porosity p and the amount of TiH2 Vb for the three types of ADC12 and A1050. When the amount of the blowing agent (TiH2) varied from 0 to 0.6 mass%, the porosity and the pore structure varied markedly with increasing amount of TiH2. From this finding, it is considered that, by varying the amount of TiH2 from 0 mass% to 0.6 mass% for ADC12 and A1050, the porous aluminum with graded porosities and pore structures can be fabricated. Figures 2(a) ![]() (c) show the pore structures of the four types of ADC12 porous aluminums without TiH2. Figure 2(e) shows the relationship between average equivalent diameter dm and total amount of contained gases. In the condition without TiH2, the average equivalent diameters (dm) remained constant when the total amount of gases was larger than approximately 90 cm3/100g. From this finding, it is considered that, by using ADC12 with the contained gases above 90 cm3/100g, the porous aluminum with different porosities and almost same average diameter of pores can be fabricated.
(c) show the pore structures of the four types of ADC12 porous aluminums without TiH2. Figure 2(e) shows the relationship between average equivalent diameter dm and total amount of contained gases. In the condition without TiH2, the average equivalent diameters (dm) remained constant when the total amount of gases was larger than approximately 90 cm3/100g. From this finding, it is considered that, by using ADC12 with the contained gases above 90 cm3/100g, the porous aluminum with different porosities and almost same average diameter of pores can be fabricated.
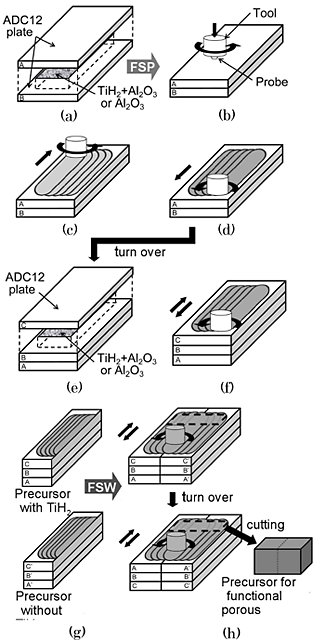
On the basis of above results, the two types of ADC12 porous aluminums (type I and type II) changing the porosities and pore structures were tried to fabricate. Figure 3 shows a schematic illustration of the fabrication process of the precursor for porous aluminum with graded porosities and pore structures. For the porous aluminum type I, ADC12 with the contained gases of 195.2 cm3/100g were used as the starting material. TiH2 powder (![]() 45
45 ![]() m, 1 mass%) and Al2O3 powder (
m, 1 mass%) and Al2O3 powder (![]() 1
1 ![]() m, 5 mass%) as the stabilization agent were both used for one of the laminated plates and only Al2O3 powder was used for the other laminated plates (Fig. 3(a)). For the porous aluminum type II, the two types of ADC12 with the contained gases of 92.0 cm3/100g and 250.6 cm3/100g were used as the starting material. Only Al2O3 powder was used for the laminated plates (Fig. 3(a)).The multipass FSP was applied to obtain large precursors and to mix the gases and powders thoroughly (Fig. 3(b)
m, 5 mass%) as the stabilization agent were both used for one of the laminated plates and only Al2O3 powder was used for the other laminated plates (Fig. 3(a)). For the porous aluminum type II, the two types of ADC12 with the contained gases of 92.0 cm3/100g and 250.6 cm3/100g were used as the starting material. Only Al2O3 powder was used for the laminated plates (Fig. 3(a)).The multipass FSP was applied to obtain large precursors and to mix the gases and powders thoroughly (Fig. 3(b) ![]() (f)). The obtained two laminated plates were cut in the stirred region (Fig. 3(g)) and were welded by conducting friction stir welding (FSW) on both sides (Fig. 3(h)). A precursor sample was cut from the stirred region including the bonding interface and then heated in a preheated electric furnace. The porous aluminums of 15 mm
(f)). The obtained two laminated plates were cut in the stirred region (Fig. 3(g)) and were welded by conducting friction stir welding (FSW) on both sides (Fig. 3(h)). A precursor sample was cut from the stirred region including the bonding interface and then heated in a preheated electric furnace. The porous aluminums of 15 mm![]() 15 mm
15 mm![]() 29 mm were cut by electro-discharge machining from the foamed samples. The pore structures in the porous aluminums were observed by X-ray CT nondestructively.
29 mm were cut by electro-discharge machining from the foamed samples. The pore structures in the porous aluminums were observed by X-ray CT nondestructively.
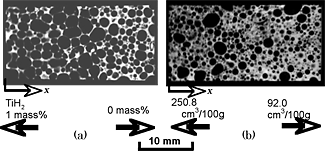
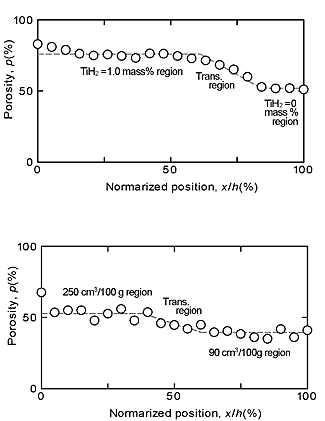
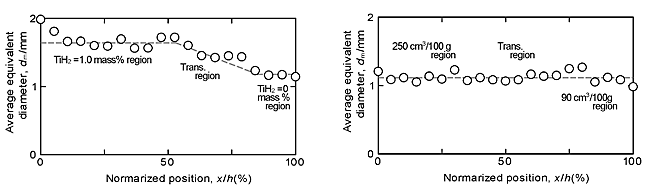
Figures 4(a) and (b) show the X-ray CT images of the porous aluminums type I and type II, respectively. Figures 5(a) and (b) show the relationships between the locations x from the left side (Fig. 4), which was normalized by the length of porous aluminum (h=29 mm), and the porosities for the porous aluminums type I and II, respectively. Figures 6(a) and (b) show the relationships between the normalized locations x/h and the average equivalent diameters of pores for porous aluminums type I and II, respectively. From these figures, it can be seen that, in the porous aluminum type I, the porosities and equivalent diameters of pores in the TiH2= 1.0 mass% region were higher than those in the TiH2=0 mass% region and changed gradually in the transition region. On the other hand, in the porous aluminum type II, although the equivalent diameter of pores were almost constant, the porosities in the 250.6 cm3/100g region were higher than those in the 92.0 cm3/100g region and changed gradually in the transition region.
From these results, it was shown that the two types of ADC12 porous aluminums with graded porosities and pore structures were practically fabricated by using the FSP route precursor method. One of the porous aluminums had two different porosities and average diameters of pores, and the other had two different porosities with almost same average diameter of pores.