Metallurgical Abstracts on Light Metals and Alloys vol.56
Effect of Propanol on Growth Rate of Anodic Porous Alumina in Sulfuric Acid
Hidetaka Asoh, Haruka Kadokura, Ryohei Murohashi and Mikimasa Matsumoto
Department of Applied Chemistry, Kogakuin University
[Published in J. Electrochem. Soc., Vol. 169 (2022), 073510]
https://doi.org/10.1149/1945-7111/ac80d5
E-mail: asoh[at]cc.kogakuin.ac.jp
Key Words: Anodization, Aluminum, Porous-type anodic film, Film formation efficiency
Recently, we have investigated the anodization of aluminum in popular acidic electrolytes (e.g., sulfuric and oxalic acids) containing an alcohol as an additive with a focus on film formation efficiency and the hardness of the anodic films. In this study, to confirm whether the effects of alcohol on anodization behavior and anodic film growth are universal regardless of the type of alcohol, we focused on the difference in carbon number of the monohydric alcohols and selected propanol (n = 3 for C3H7OH) with a three carbon chain (C3-alcohol) as an additive. Here, sulfuric acid was used as the electrolyte with concentrations of 0.3 and 1.5 mol‧dm−3. Propanol has two isomers, i.e., 1-propanol with the OH group on an end carbon and 2-propanol with the OH group on the middle carbon; thus, the difference of isomer on film formation was also investigated with a focus on the physical properties (e.g., viscosity and electric conductivity) of the mixed solution, the steady state voltage, and the anodic film’s growth rate. Adding propanol improved the film growth rate and current efficiency unlike sulfuric acid only, even under high current density conditions. However, there was no clear difference in the effect of different isomers on film growth rate.
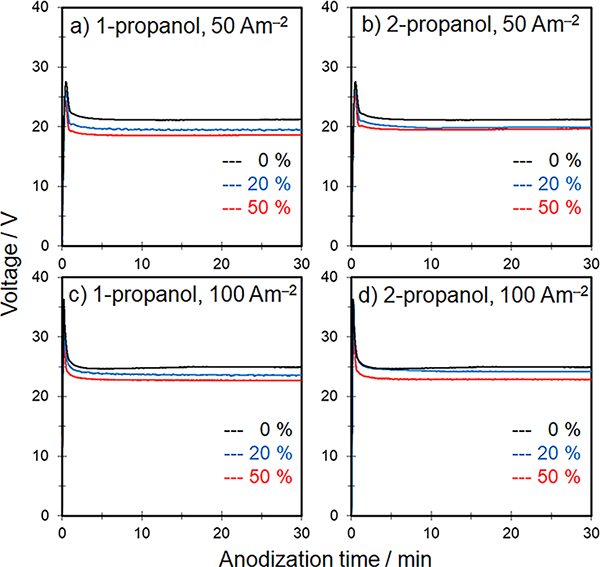
The steady state voltage during constant current anodization decreased with an increasing amount of propanol regardless of the isomer type and the current density.
