Metallurgical Abstracts on Light Metals and Alloys vol.56
Chemical Conversion Treatment of AA5083 Aluminum Alloy and AISI 1045 Carbon Steel under Galvanically Coupled Condition in Na2MoO4: Effect of pH on Corrosion Resistance
Takumi Kosaba*, Izumi Muto*, Masashi Nishimoto* and Yu Sugawara*
*Department of Materials Science, Graduate School of Engineering, Tohoku University
[Published in Materials Transactions, Vol. 64 (2023), pp. 568-577]
https://doi.org/10.2320/matertrans.MT-M2022163
E-mail: mutoi[at]material.tohoku.ac.jp
Key Words: Conversion treatment, Sodium molybdate, Galvanic corrosion, AA5083, Carbon steel
To ascertain the effect of solution pH of Na2MoO4 chemical conversion treatment for aluminum/steel joints on corrosion resistance, AA5083 aluminum alloy and AISI 1045 carbon steel were immersed in 50mM Na2MoO4 at pH ranges of 8-12 under galvanically coupled condition. Subsequently, in diluted synthetic seawater, the galvanic corrosion resistance of the AA5083 alloy connected to the AISI 1045 carbon steel was assessed. The number of localized corrosion damages was counted, and AA5083 treated at pH 11 was found to be the better corrosion resistance. The oxygen reduction current on bulk Al6(Fe, Mn) decreased with increasing solution pH of the conversion treatment. The Al6(Fe, Mn) particles on AA5083 were not preferential cathodes, and alkalization through oxygen reduction would not occur when the treatment was performed above pH 9. Auger electron spectroscopy analysis showed that Mo-accumulation, Fe-removal, and film thickening occurred on the particles of AA5083 treated at pH 11. These factors contributed to the suppression of the cathodic activity of the Al6(Fe, Mn) particles, resulting in the improved galvanic corrosion resistance of AA5083.
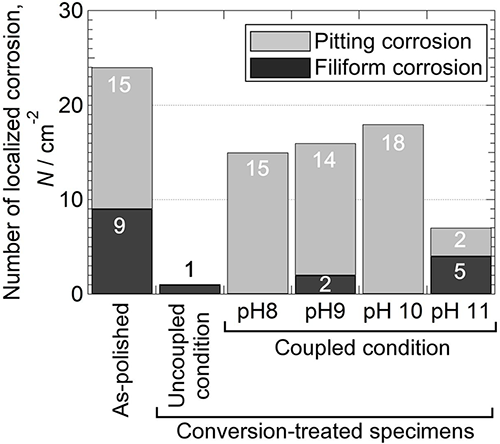
Effect of solution pH used in the conversion treatment on the number of localized corrosion damages on as-polished and conversion-treated AA5083 after galvanic current and potential measurements.
