Metallurgical Abstracts on Light Metals and Alloys vol.56
Role of KMnO4—NaF Treatment in Galvanic Corrosion Resistance of AA5083 Coupled to Steel
Takumi Kosaba*, Izumi Muto*, Masashi Nishimoto* and Yu Sugawara*
*Department of Materials Science, Graduate School of Engineering, Tohoku University
[Published in Materials Transactions, Vol. 64 (2023), pp. 896-903]
https://doi.org/10.2320/matertrans.MT-L2022021
E-mail: mutoi[at]material.tohoku.ac.jp
Key Words: Galvanic corrosion, AA5083, Carbon steel, Chemical conversion, Potassium permanganate
The role of KMnO4—NaF conversion treatment in the galvanic corrosion resistance of AA5083 aluminum alloy coupled to AISI 1045 carbon steel in synthetic seawater (diluted 100 times) was investigated. The 10 min-treated AA5083 was observed to reduce the period of high current density during the early stage of coupling and decrease the number of localized corrosion damages on the AA5083. The 10 min conversion treatment significantly reduced the electrode potential of bulk Al6(Fe, Mn), and it was concluded that the Al6(Fe, Mn) particles on the conversion-treated AA5083 no longer acted as a local cathode at the electrode potential when the AA5083 was in contact with AISI 1045 carbon steel. The decrease in cathodic activity of Al6(Fe, Mn) was attributed to the removal of Fe from the surface film of Al6(Fe, Mn), addition of Mn by the conversion treatment, and thickening of the film.
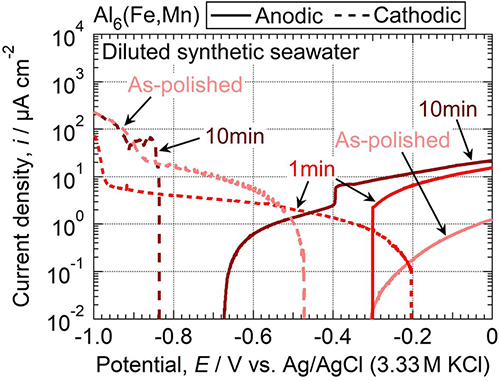
Potentiodynamic anodic and cathodic polarization curves of as-polished and conversion-treated bulk Al6(Fe, Mn) in the naturally aerated diluted synthetic seawater at pH 8.2 (298 K). The solid and dashed lines indicate the anodic and cathodic polarization curves, respectively.
