Metallurgical Abstracts on Light Metals and Alloys vol.56
Change in Oxygen Reduction Reactivity of Intermetallics: A Mechanism of the Difference in Trenching around Al-Fe and Al-Fe-Si Particles on AA1050 in NaCl
Hiroshi Kakinuma*, Izumi Muto*, Yoshiyuki Oya**, Takahiro Momii**, Ying Jin***, Yu Sugawara* and Nobuyoshi Hara*
*Department of Materials Science, Tohoku University
**Research & Development Division, UACJ Corporation
***National Center for Material Service Safety, University of Science & Technology Beijing
[Published in Journal of The Electrochemical Society, Vol. 170 (2023), 021503]
https://doi.org/10.1149/1945-7111/acb6ba
E-mail: mutoi[at]material.tohoku.ac.jp
Key Words: AA1050, pitting corrosion, trenching, Intermetallics
Trenching around intermetallic particles on AA1050 aluminum in 0.1 M NaCl at pH 6.0 was analyzed by in situ observations. Deep trenches, which become the initiation site for pitting, were formed around the Al-Fe-Si particles but not around the Al-Fe particles. The open-circuit potentials of the bulk intermetallic compounds and Al-matrix of AA1050 without intermetallic particles were measured. It was determined that the Al-Fe-Si and Al-Fe particles were cathodically polarized by the Al-matrix of AA1050 under natural immersion conditions. This cathodic polarization was found to change the oxygen reduction reactivity of the intermetallic particles. The cathodic reactivity on bulk Al-Fe was higher than that on bulk Al-Fe-Si under as-polished condition. However, after potentiostatic cathodic polarization, the cathodic reactivity on bulk Al-Fe decreased, whereas that on bulk Al-Fe-Si increased. Micro-electrochemical measurements and surface analyses clarified that the change in the cathodic reactivity of the intermetallic particles plays a critical role in trenching on AA1050 aluminum.
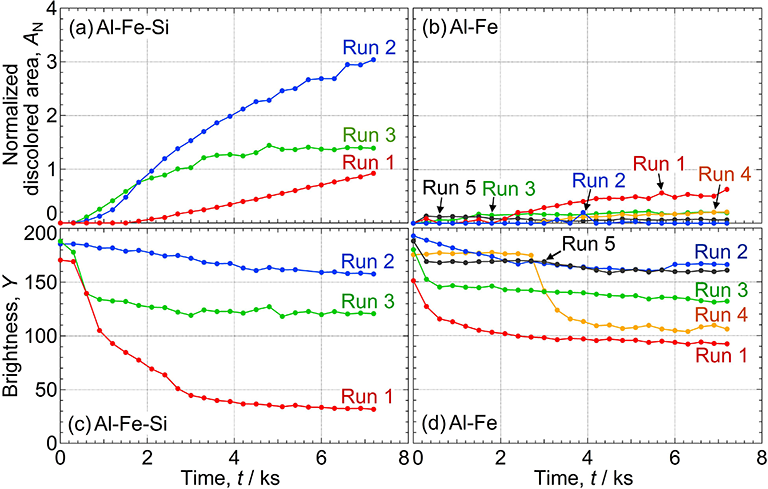
Time variations of the discolored area of the Al matrix around (a) Al-Fe-Si and (b) Al-Fe particles and the Y value of (c) Al-Fe-Si and (d) Al-Fe particles during the OCP measurements in 0.1 M NaCl (pH 6.0).
