Metallurgical Abstracts on Light Metals and Alloys vol.56
Effect of Solution Temperatures on Electrochemical Behavior of Aluminum Alloys and Carbon Steel in 20 Mass% NaCl Solutions
Tong Shen* and Masatoshi Sakairi**
*Graduate School of Engineering, Hokkaido University
**Faculty of Engineering, Hokkaido University
[Published in Zairyo-to-Kankyo, Vol. 71 (2022), pp.138-142]
https://www.jstage.jst.go.jp/article/jcorr/71/5/71_138/_article/-char/en
E-mail: msakairi[at]eng.hokudai.ac.jp
Key Words: aluminum alloys, carbon steel, sub-zero temperature, electrochemical impedance spectroscopy
Effects of temperature on electrochemical behavior of aluminum alloys and carbon steel were investigated in aerated 20 mass% NaCl solutions at sub-zero temperatures. Rest-potentials of carbon steel shifted to positive direction with decreasing temperature, which of aluminum alloys were unaltered with temperature. Clear oxygen diffusion limiting current densities were observed in all the experimental conditions. The limiting current changes are in the order of A6061 < A1050 < carbon steel. The oxygen limiting currents of carbon steel decreases slightly with decline of temperature, which of Al alloy are independent of temperature. Electrochemical impedance spectroscopy (EIS) results of carbon steel implies that EIS behavior changes with temperature.
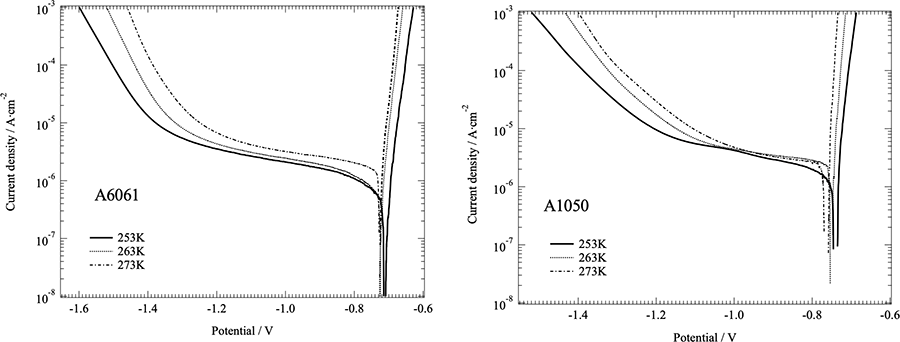
Polarization curves of A6061 and A1050 in 20 mass% NaCl solutions at different temperature.
